Gabriele Curci
Atmospheric composition by large impacts

Gabriele CurciAtmospheric composition by large impacts |

|
 |
For a full account on this work, please refer to: Curci, G., G. Visconti, D. J. Jacob and M. J. Evans (2004), Tropospheric fate of Tunguska generated nitrogen oxides, Geophys. Res. Let., L06123, doi:10.1029/2003GL019184. [Abstract (HTML)] [Full text (PDF)] This work has also been presented at EGU 2004: Tropospheric fate of Tunguska generated nitrogen oxides, EGU04-A-02116 in Session AS3.03: Trace gases in the atmosphere: observations and modelling, EGU 1st General Assembly, Nice, France, 29 April 2004. [PowerPoint Presentation] News 22/07/2008: This story was covered by National Geographic News! (Read until page 2 ;-) ) |
On the morning of 30th June 1908 an extraterrestrial body entered the Earth's atmosphere and exploded at an altitude of about 9 km. The energy released at the moment of the blast was of about 5 × 1016 J, that is 60 times the energy of the Hiroshima atom bomb. The enormous heat generated by the explosion burnt 2000 km2 of forest and seared to death a farmer. A bright fireball was seen in the sky 170 km away and the pressure shock was recorded by seismographs at a distance of 1000 km.
We studied the consequences of such event from the point of view of the atmospheric chemist. When the air is heated to temperatures above 2000° K, e.g. in air nearby a lightning discharge, a power plant stack or a falling asteroid, the molecules of nitrogen (N2) and oxygen (O2), the two most abundant species in our atmosphere, dissociate and react to form nitric oxide (NO):
| O2 | =heat=> | O + O |
| O + N2 | ==> | NO + N |
| N + O2 | ==> | NO + O |
Once emitted in the atmosphere, NO reacts with other gases and forms NO2. NO and NO2 together form the NOx family, that play a key role in the chemistry both of the troposphere and of the stratosphere. NO2 can react with hydroxyl radical (OH) to form nitric acid (HNO3) that can be lost to the ground as acid rain because of its high solubility in water.
The Tunguska explosion may have released about 0.4 Tg (1 Tg = 1012 g) of NO in the upper troposphere. We have used a 3-D model of chemistry and transport (GEOS-CHEM model, Harvard University) to simulate how the atmosphere reacts to a sudden injection of such an amount of nitric oxide, how long it takes for the atmosphere to recover from the shock and which damage to the ecosystem are to be expected because of the acid deposition induced.
We start our simulation on July 1st 1998 distributing 0.4 Tg of NO between 5 km and the tropopause (located at about 8 km) in the grid-boxes corresponding to Tunguska explosion location (61° N, 102° E). We are not trying to reconstruct exactly the dynamics of year 1908, so that the generic year 1998 it's fine to show our point. We are neither introducing perturbations to temperature and pressure fields due to the explosion. The background emissions are also relative to 1998: the NO perturbation introduced is so large in comparison to the background that an exact reconstruction of the latter is of little importance. Let's now look at what happens to the shock-generated nitrogen in the following animated GIFs.
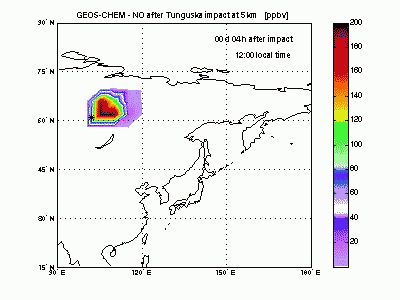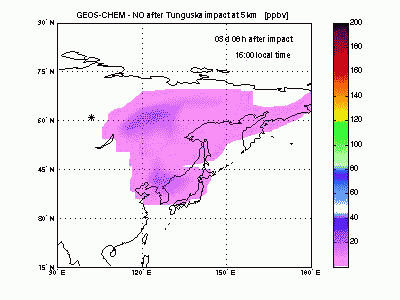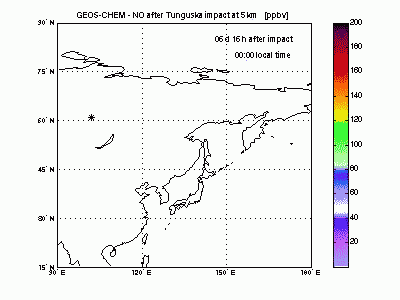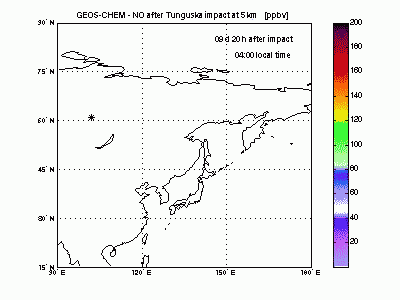
Nitric OxideThe nearby animation shows how nitric oxide mixing ratio (ppbv) evolves in the model at 5 km altitude. The star on the map indicates where Tunguska object explosion took place. The NO plume generated by the explosion moves eastward transported by the winds and after 1 day is already 2000 km away from the blast point. The mixing ratio quickly decreases partly because of dilution with background air and partly because of conversion to NO2. After 2-3 days the initial mixing ratio of NO is already greatly reduced, and after then the local mixing ratio peaks significantly on top of atmospheric background only in the early morning. |
 |
OzoneLet's look at ozone mixing ratio (ppbv) at 5 km. Soon after impact the ozone is locally wiped out by the NO that in this way produce NO2. Ozone destruction by NO is visible in the animation as the blue-purple “hole” correlated to the high-NO plume just seen above. When the shock-generated NO is largely depleted it is not effective anymore in scavenging ozone. Indeed, toward the end of the animation, it can be seen how NOx start to catalyze production of ozone (dark red mass at 150° E). |
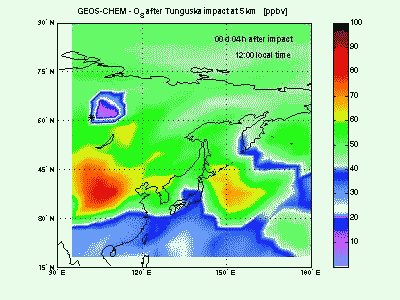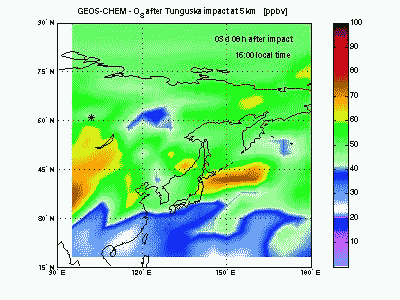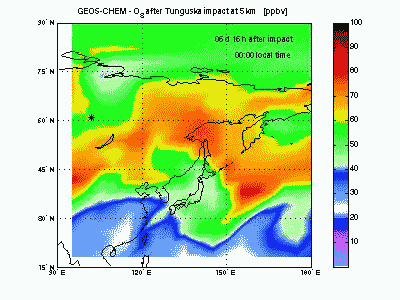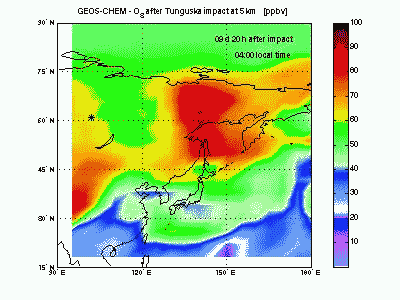 |
Nitric DioxideShock-generated NO undergoes fast conversion to NO2. In the animation it can be seen how an NO2 plume forms and moves eastward and how it gradually dissipates because of formation of HNO3 by reacting with OH. After 4-5 days shock-derived NO2 peaks only in late afternoon, when the sun is down and all the NO is converted to NO2. |
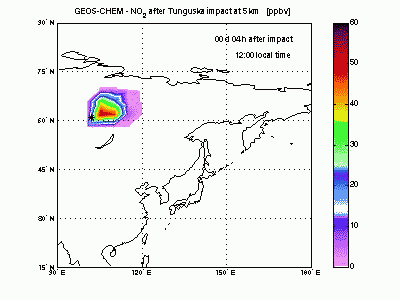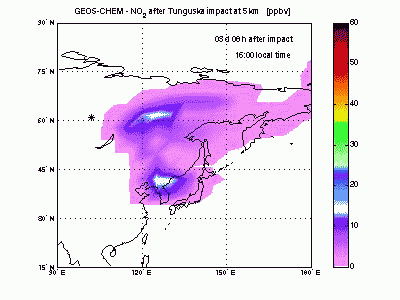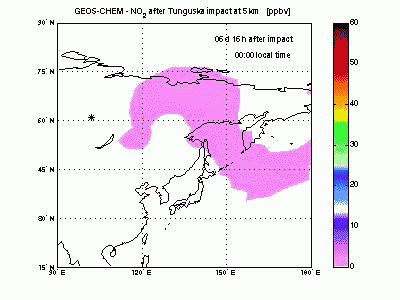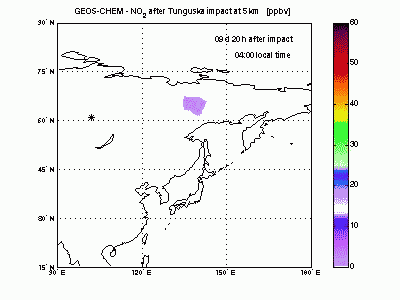 |
Hydroxyl RadicalSimilarly to ozone, the hydroxyl radical (OH) is scavenged in the air entrained with shock-derived NO2 and forms nitric acid (HNO3). NO2 conversion to HNO3 takes place only during the day, because at night OH cannot be produced from ozone photolysis and NO2 is rapidly converted into other nitrogen oxides (NO3, N2O5...). A sharp hole in the daytime OH field can be observed in the movie during the first 3-4 days after impact. After then OH concentration peaks in correspondence to the region of ozone production by shock-generated NOx. |
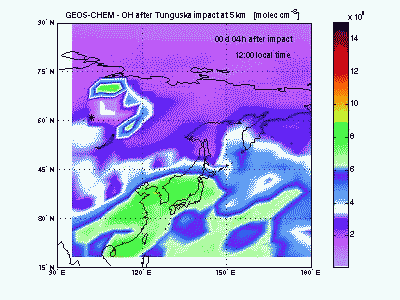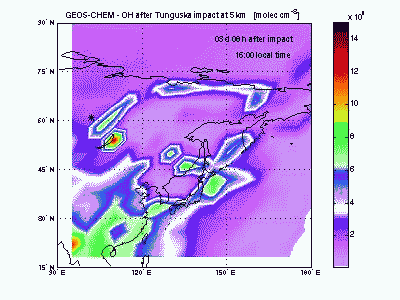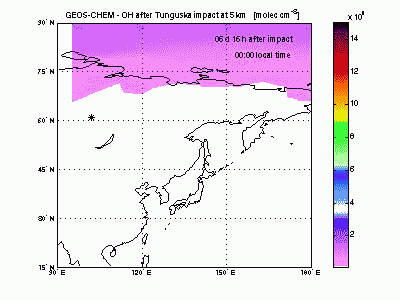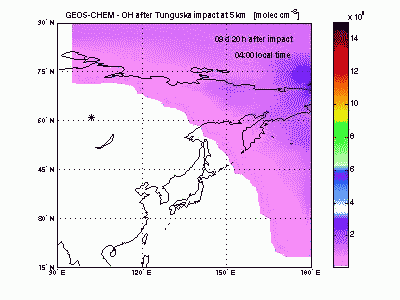 |
Nitric AcidHNO3 is produced from reaction of NO2 with OH. Its lifetime against chemical destruction is much longer than nitrogen oxides and its main removal process is wet deposition, because of its high solubility in water. We found that about 80% of HNO3 formed after Tunguska explosion is lost by wet deposition, with the remaining 20% lost by dry deposition. The acid deposition in the region downwind the blast point may have induced rain pHs as low as 4 during the first month after the explosion. These values of pH are comparable to those found today nearby industrialized zones, but the acid deposition is not expected to have caused large damages to the ecosystem because of its short duration. |
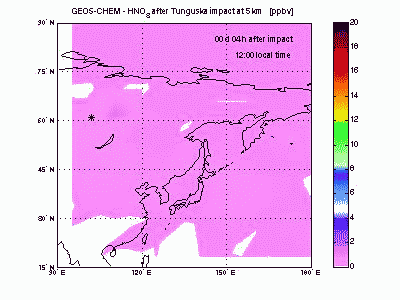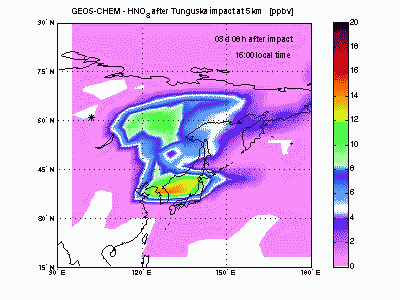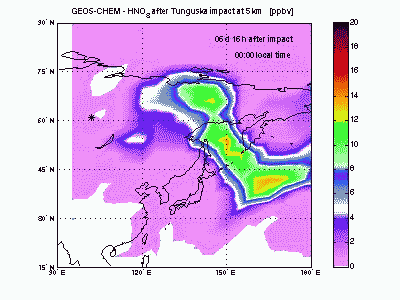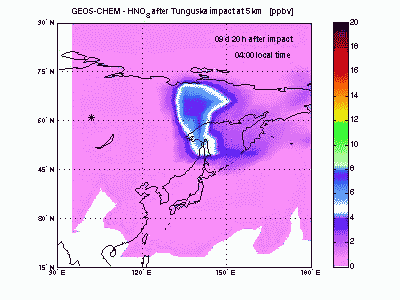 |
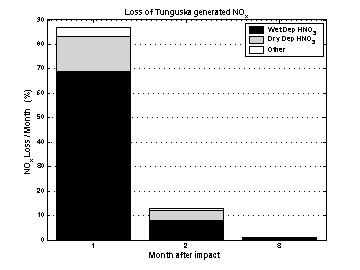 |
Nitrogen lossThe histogram shows the fraction of shock-generated nitrogen lost during the first three months of after simulated Tunguska impact. The 87% of nitrogen is lost after one month and almost all the remaining is lost during the second month. The bar subdivisions show how different loss processes contribute to total loss. As can be seen, wet deposition of HNO3 is largely the main removal process, followed in importance by dry deposition of HNO3. Deposition of other nitrogen containing compounds is negligible. |
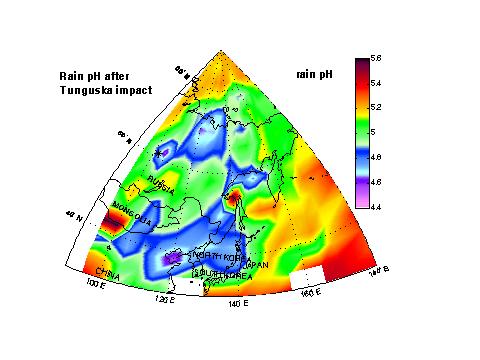
Rain pHHNO3 that absorbs in raindrops lowers rain pH. The figure shows how shock-derived nitric acid lowers rain pH from simple CO2-H2O equilibrium during the first month of our simulation. Calculated pHs are always higher than 4. Those pH values are comparable to those found nearby modern industrial areas, but Tunguska acid deposition is not expected to produce large damages to the ecosystem because of its short duration (about 1 month). |
 |
Tunguska LinksPicturesque eye-witness reconstruction of 1908 Tunguska eventTunguska home page at University of Bologna Curiously 1908 is the same year of foundation of my favourite soccer team... |